Friday, 1 July 2016
Thursday, 26 May 2016
Saturday, 26 March 2016
Cement Industry
Cement Industry is very important in economy of a country. It play an important role in the building materials of country.
Here are slides on cement industry available on following link
Friday, 4 March 2016
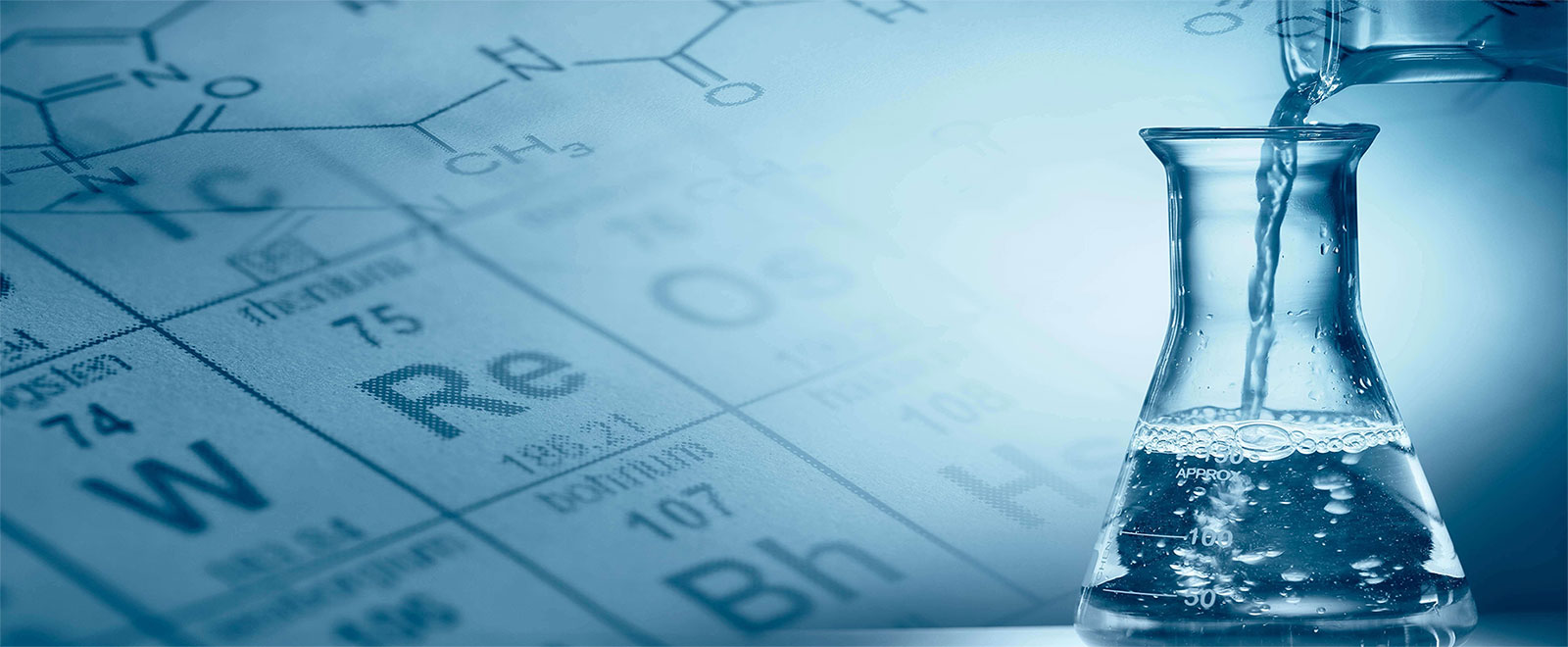
Water Treatment
Water is a universal solvent
Water is a universal solvent. It has ability to dissolve some organic and mostly inorganic materials.water is very important for the existence of life on earth.Major part(about 75%) of our earth is contained by water.
Uses
Water is used by every live thing on earth. For example, plants consume water to grow and prepare their food.
Animals get water through rivers, and open water resources.
Human beings use water for drinking, bathing, in industries.
As stated earlier water can dissolve at most every material. It can dissolve impurities and hazardous chemicals in it.
To prepare pure water, it water is treated through different procedures.
Following link has PDF file on water treatment:
Hydrostatic Equilibrium In Centrifugal Field
Centrifugal field is also an application of Hydrostatic Equilibrium it works on the fact that the more the fluid dense, more it farther from the axis of rotation .The lighter (with comparatively low density) liquid will form a layer on heavy liquid.
In a rotatory centrifuge the liquid is thrown outward from the axis of rotation. Surface above the liquid take a shape of a curve or parabolic shape when the centrifuge is rotated about its axis.But at very high speed(In industrial centrifuge) it is rotated at very high speed & surface above liquid take a shape of cylindrical layer.
r1 is the radial distance from the axis of rotation to the free liquid surface.
r2 is the radius of the centrifugal bowl.
The whole mass of the liquid rotates like a rigid body.
F = ma.........(1)
Taking Differential
dF = adm......(2)
The acceleration of the fluid is given as; a = ω 2 r
Substituting a = ω 2 r in eq (1) becomes
dF = ω 2 rdm......(3)
If ρ is the density of the given liquid, and b is the breadth of the ring, thus, the mass of the element can be written as; m = πr2 b
differentiating equation (3) leads to dm = d(πr2 b · ρ) = 2πrbρ · dr
Final equation of Centrifugal field become
In a rotatory centrifuge the liquid is thrown outward from the axis of rotation. Surface above the liquid take a shape of a curve or parabolic shape when the centrifuge is rotated about its axis.But at very high speed(In industrial centrifuge) it is rotated at very high speed & surface above liquid take a shape of cylindrical layer.
r1 is the radial distance from the axis of rotation to the free liquid surface.
r2 is the radius of the centrifugal bowl.
The whole mass of the liquid rotates like a rigid body.
F = ma.........(1)
Taking Differential
dF = adm......(2)
The acceleration of the fluid is given as; a = ω 2 r
Substituting a = ω 2 r in eq (1) becomes
dF = ω 2 rdm......(3)
If ρ is the density of the given liquid, and b is the breadth of the ring, thus, the mass of the element can be written as; m = πr2 b
differentiating equation (3) leads to dm = d(πr2 b · ρ) = 2πrbρ · dr
Final equation of Centrifugal field become
Applications of Hydrostatic Equilibrium
When we study some thing we look for its application in our daily day life. The hydrostatic equilibrium has wide applications in engineering & other fields of science.
Some Applications of Hydrostatic Equilibrium are here
- Barometric Equation
- Manometers
- Centrifuge & Decanters
Barometric Equation states that Pressure(P) decreases with increase in Height(h)
It is given as follow:
P=Cexp(−MgRTh).
Derivation
Considering an ideal gas with density through pressure P then according to ideal gas law: PV=mMRT,⇒P=mVMRT=ρMRT. Thus, dependency of the barometric pressure on the altitude is given by the formula If the pressure is given in millimeters of mercury |
Example 1
|
Determine at what altitude the air pressure is twice less than on the sea level?
Solution. |